millon's test for protein|Millon's reagent : Cebu Principle of Millon’s test: Compounds containing hydroxybenzene radical react with Millon’s reagent to form red complexes. The only amino acid having hydroxybenzene ring is tyrosine. Thus, this test is . XNXX.COM 'literotica' Search, free sex videos. Language ; Content ; Straight; Watch Long Porn Videos for FREE. Search. Top; A - Z? This menu's updates are based on your activity. The data is only saved locally (on your computer) and never transferred to us. . Results for : literotica. STANDARD - 26 GOLD - 26.
PH0 · Test of Proteins Experiment
PH1 · Organic Chemistry: Lesson 19. QUALITATIVE TEST FOR
PH2 · Millon’s test: Principle, Reaction, Reagents, Procedure
PH3 · Millon’s test: Objective, Principle, Reagents,
PH4 · Millon’s test – Its Principle, Reagents, Procedure etc
PH5 · Millon’s Test: Objective, Principle, Procedure And Result
PH6 · Millon’s Test
PH7 · Millon's test
PH8 · Millon's reagent
PH9 · Methods for Rapid Screening of Biologically Active Compounds
Thank you for your message and for reaching out to us. For immediate concerns, such as lost or stolen BPI debit and credit cards, please call our 24-hour BPI Contact Center at (+632) 889-10000 for Metro Manila or 1-800-188-89100 for domestic toll-free calls.
millon's test for protein*******Millon’s test is an analytical test used for the detection of the amino acid tyrosine, which is the only amino acid containing the phenol group. Millon’s test is a specific test for tyrosine, but it is not a specific test for protein as it also detects the phenolic group present in other compounds as well. Therefore, . Tingnan ang higit paMillon’s test is based on the principle of nitrification of the phenol group in tyrosine, which then forms complexes with heavy metals like mercury. The reagent used for the test is called Millon’s reagent, and it consists of mercuric nitrate and mercurous nitrate that . Tingnan ang higit pa Millon’s test is specific test for identification of tyrosine. Tyrosine containing protein when reacts with acidified mercuric sulphate solutions gives yellow precipitate of mercury protein complex. Millon’s test is an analytical test used for detection of presence of soluble proteins. Millon’s test is one of the qualitative methods to determine differences in types of proteins and .
Principle of Millon’s test: Compounds containing hydroxybenzene radical react with Millon’s reagent to form red complexes. The only amino acid having hydroxybenzene ring is tyrosine. Thus, this test is .Millon's reagent is an analytical reagent used to detect the presence of soluble proteins. A few drops of the reagent are added to the test solution, which is then heated gently. A reddish-brown coloration or precipitate indicates the presence of tyrosine residue which occur in nearly all proteins. The test was developed by the French chemist Auguste Nicolas Eugene Millon.Millon’s test is used for the detection of amino acid tyrosine. REAGENTS: Millon’s Reagent: Mixture of mercurous and mercuric nitrates. PRINCIPLE: The mercurous and mercuric nitrate .Millon’s test is given by proteins containing phenolic amino acids. Gelatin does not give this test. First white precipitate is formed when proteins treated with millon reagent and turns to brick red on boiling confirms the presence of .
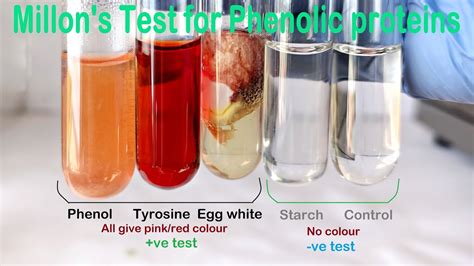
Millon’s test (proteins)–white precipitate, which turns red after heating, indicates the presence of proteins [ 28, 37] and free amino acids [ 34, 39 ]. This method turned out to be . Millon’s test is a specific test for tyrosine, but it is not a specific test for protein as it also detects the phenolic group present in other compounds as well. Therefore, while .Millon’s reagent. 19.2.1.3 Method. Add 3 to 4 drops of Millon’s reagent to 5 ml of test solution. Mix and bring the mixture gradually to a boiling point by heating over a small flame. Development . Result and Interpretation of Millon’s Test. Positive result:A red or pink-tinted precipitate forming during the Millon’s test is indicative of a favourable outcome. This indicates the presence of tyrosine or a protein having tyrosine. Negative result:The lack of coloured precipitate in the test tube shows that the Millon’s test was . The term ‘Xantho’ refers to ‘yellow’, so the test is often termed as the Yellow Protein Test. The test gives a positive result for amino acids containing benzene rings or other aromatic groups. The test is a qualitative . Plants, due to the large quantities of carbohydrates that make up their structure, contain lower amounts of protein compared to animal cells . However, the vegetal food production has a lower environmental footprint than animal husbandry. . Millon’s test (for proteins)–Millon’s reagent (2 mL) was added to the extract (2 mL) and heated .
Test : Add 1ml of Millon's reagent to 1-2ml of test solution. If desired heat the solution till it boil. Results : Presence of proteins (tyrosine, the only amino acid containing a phenol group) absence of protein Storage and Shelf Life Store between 10-30°C in tightly closed container and away from bright light. Use before expiry date on label. On
Experiment 1: Test for Protein – Millon’s experiment Time allocation: 45 Min Millon’s test utilize a reagent called Millon’s test solution which is a solution of mercuric and mercurial ions in nitric and nitrous acid. The hydroxyl group (free-OH) reacts with tyrosine (an .
principle of testing tyrosine with the Millon test method is to homogenate 1-3 drops of Millon reagents into a test tube that already contains a 2% protein solution. After that, the solution is heated using a water bath and observed the changes in color and the formed deposits [15]. 2.3 Xanthoproteic testMillon’s test is given by proteins containing phenolic amino acids. Gelatin does not give this test. . If there is the appearance of bluish violet colour indicates the presence of protein. (b) Xanthoproteic Test: Take 2ml of given sample compound in a test tube. Add a few drops of concentrated sulfuric acid and heat. Hopkin’s Cole test is a specific test used for the detection of indole ring and thus, tryptophan in proteins. The test is also termed as ‘glyoxylic acid test’ as the reagent contains glyoxylic acid. The test was discovered by Frederick Gowland Hopkins and Sydney W. Cole in 1901 as a part of their work on the discovery of tryptophan.The six tests are: (1) Ninhydrin Test (2) Biuret Test (3) Xanthoproteic Test (4) Millon’s Test (5) . Chemical Reactions of Amino Acid and Protein Functional Groups: Certain functional groups in amino acids and proteins can react to produce characteristically coloured products. The colour intensity of the product formed by a particular group .millon's test for proteinThe six tests are: (1) Ninhydrin Test (2) Biuret Test (3) Xanthoproteic Test (4) Millon’s Test (5) . Chemical Reactions of Amino Acid and Protein Functional Groups: Certain functional groups in amino acids and proteins can react to produce characteristically coloured products. The colour intensity of the product formed by a particular group .
Millon's test was discovered by the French Chemist Auguste Nicolas Eugene Millon. Millon’s test is predicated on the principle of nitrification of the phenol group in tyrosine, which then forms complexes with significant metals like mercury.A reagent may be a compound or mixture added to a system to begin or check a chemical change.. A reagent may be used to . Millon's test was discovered by the French Chemist Auguste Nicolas Eugene Millon. Millon’s test is predicated on the principle of nitrification of the phenol group in tyrosine, which then forms complexes with significant .Millon's reagent Millon’s Test. It is a specific test for the detection of phenolic amino acids like tyrosine. The principle of Millon’s test is based upon the nitration reaction. Millon’s test makes the use of a nitrifying agent, i.e. concentrated nitric acid, . Millon’s test (Cole’s test) . Tyrosine containing protein when reacts with acidified mercuric sulphate solutions gives yellow precipitate of mercury protein complex. On addition of sodium nitrite solution and heating, the yellow complex of mercury phenolate forms, which is red in colour. Proteins that contain tyrosine will, therefore .Test sample, Millon’s reagent. Procedure Take 2 ml of test sample and add 2 ml Millon’s reagent in it. Then boil it for 2-5 minutes in water bath to develop red color pre-cipitate. Observation of red colored precipitates indicates presence of protein in the test solution. However, excess of reagent produces

Millon's test. Phenolic amino acids such as Tyrosine and its derivatives respond to this test. Compounds with a hydroxybenzene radical react with Millon’s reagent to form a red colored complex. Millon’s reagent is a solution of mercuric sulphate in sulphuric acid. Histidine test . This test was discovered by Knoop.millon's test for protein Millon's reagent Some of the qualitative methods that are used to detect different types of proteins and amino acids are Ninhydrin test, Xanthoproteic test, Millon's test, Sulfur test, Hopkins-Cole test, and .
Qualitative tests of amino acids 1.Solubility test: 2. Ninhydrin test for α-L amino acids 2. Xanthoproteic test for Aromatic amino acids 3. Millon's test for amino acids containig hydroxy phenyl group 4.Sakaguchi Test 5. Detection of amino acids containing sulfhydral group (- SH)/ Lead Sulfite Test
Sakaguchi Test Definition. Sakaguchi test is a biochemical test consisting of colorimetric reaction for the detection and quantification of guanidinium groups, used as a qualitative test for arginine that is either free or in protein.
Each Way Bet: Each Way (short EW) involves two bets on a single selection, splitting the total stake between a ‘win’ bet and a ‘place’ bet.This means if your selection wins, you’ll receive returns from both parts of your bet. However, if your selection only ‘places’ (finishes in the top positions but does not win), you’ll receive just the place part .
millon's test for protein|Millon's reagent